Specificity of clinical trials of medicinal products and medical devices
Clinical trials of medicinal products are conducted on each new drug that is to be placed on the market. They are also organized when examining the safety of already registered drugs or checking their effectiveness is necessary, if they are to be used in new medical indications.
On the other hand, clinical trials of medical devices, as part of clinical evaluation of a given device, are intended to verify whether the properties and performance of the device comply with the requirements according to the MDR Regulation, i.e. checking whether the operation of a given medical device is effective and safe.
Clinical trials of medical devices are, in principle, subject to the same ethical standards as clinical trials of medicinal products. However, not every medical device must undergo clinical verification – it is derived from the class of the device (see figure below).
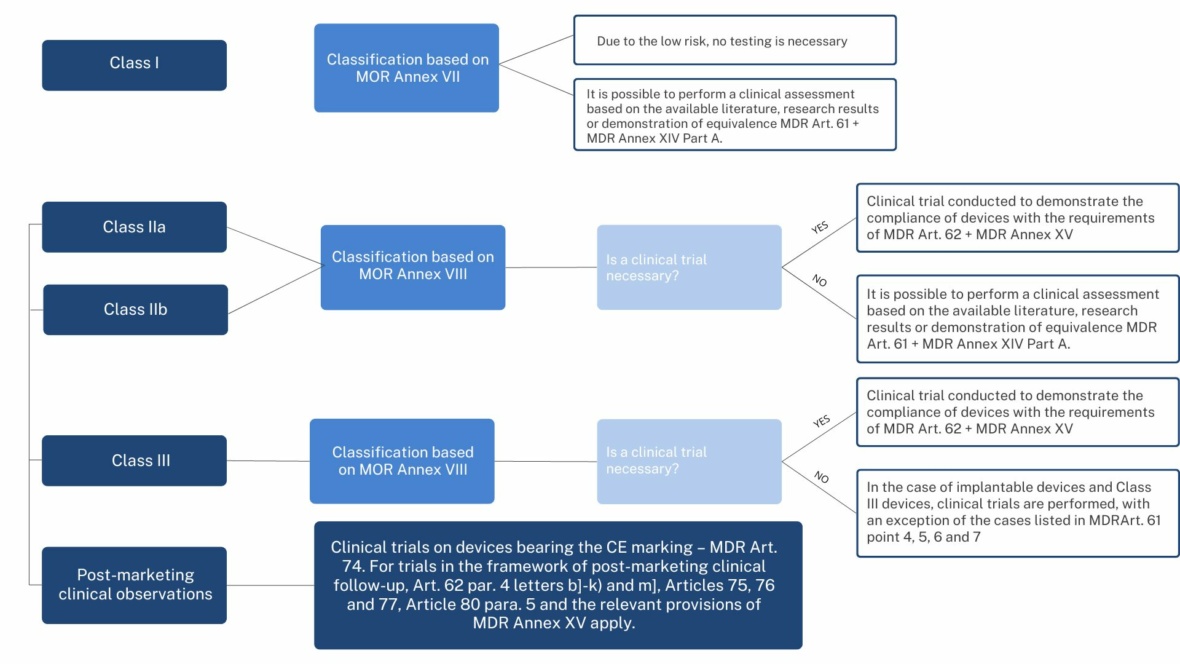
(authors of the scheme: dr hab. Marek Świerczyński, prof. UKSW, Karol Makowski Medigent LAB)
An important category among medical technologies are devices for IVD diagnostics, which, in accordance with Regulation 2017/746 of April 5, 2017, are subject to the „assessment of performance”, in practice, this means examining and analyzing data to determine or verify scientific relevance, analytical performance – and where applicable – clinical efficacy of the device.